Board
Collaborations
Technology
Parkinson’s disease
Backbone Therapy: L-dopa for 55 years
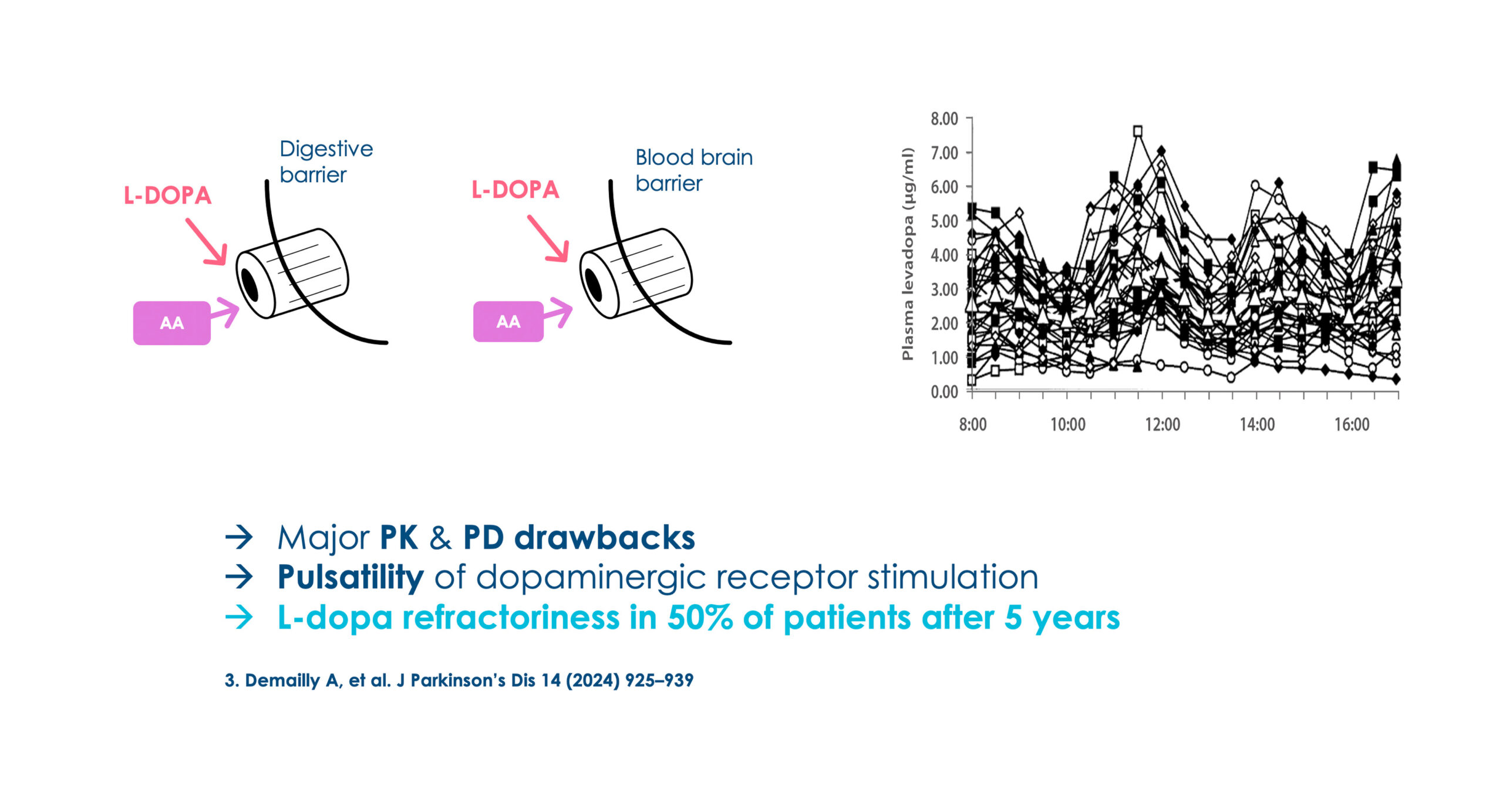
Current Device Aided Therapies (DAT) in advanced stage
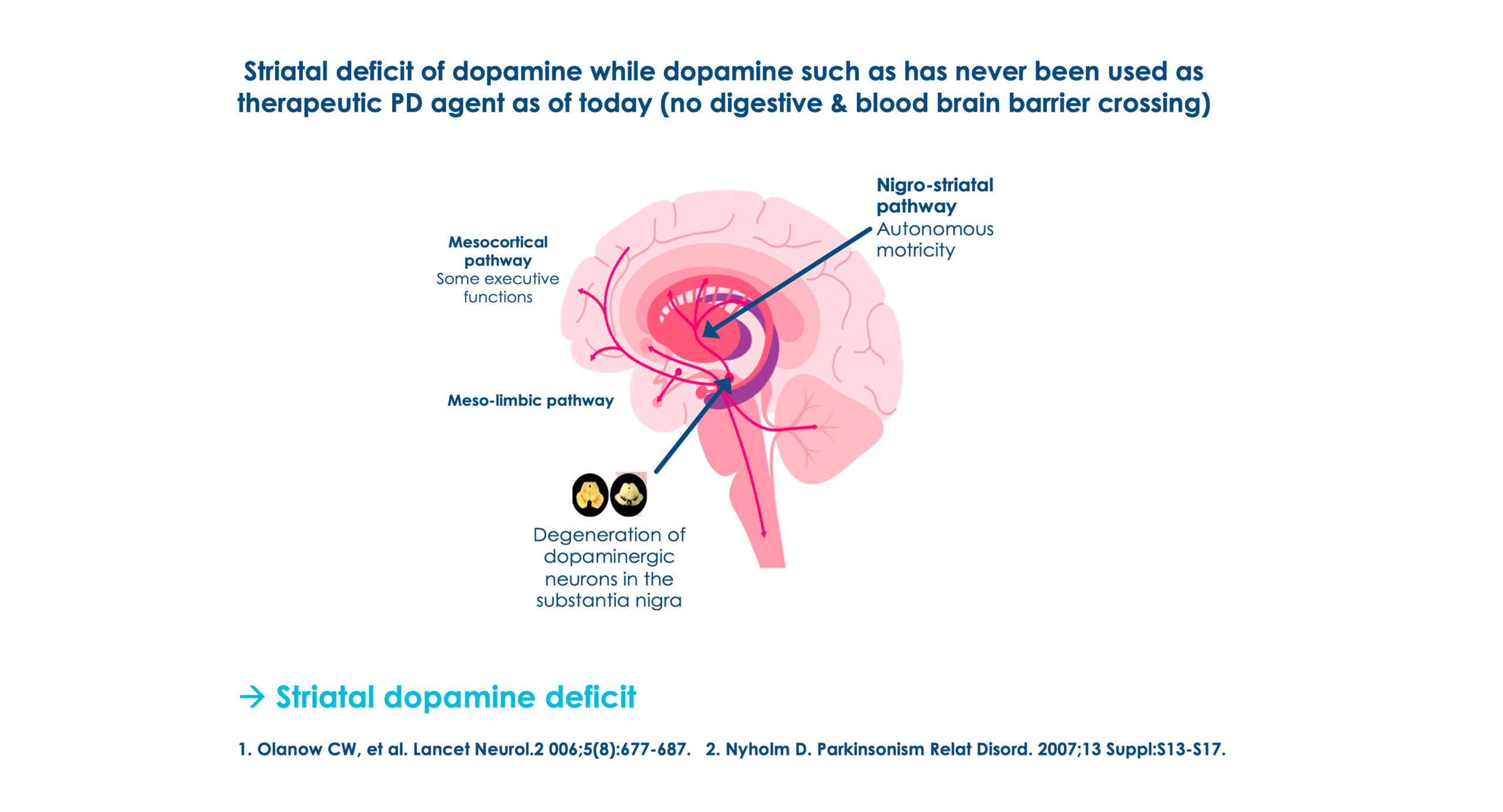
Parkinson’s Disease (PD) Etiology
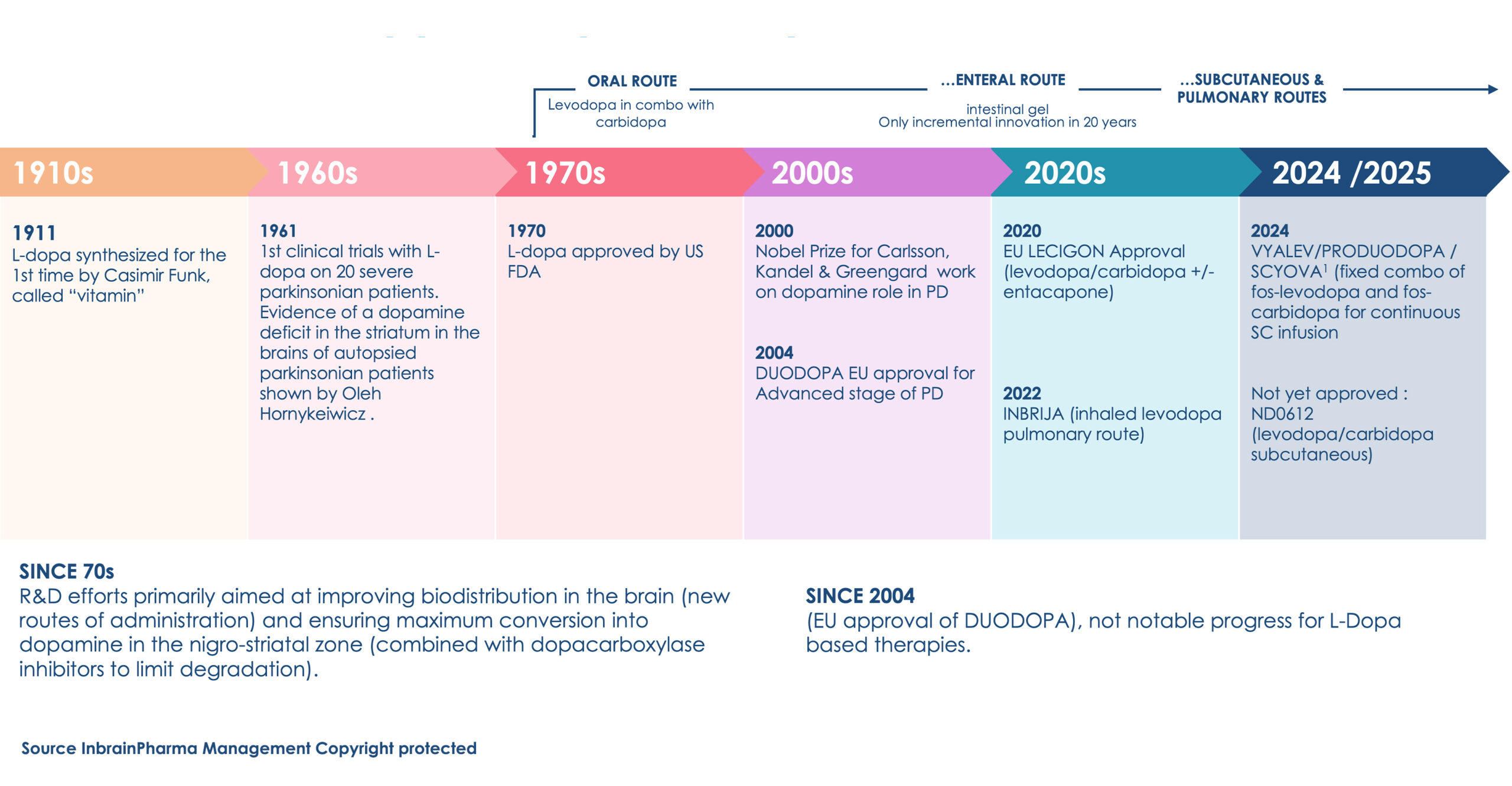
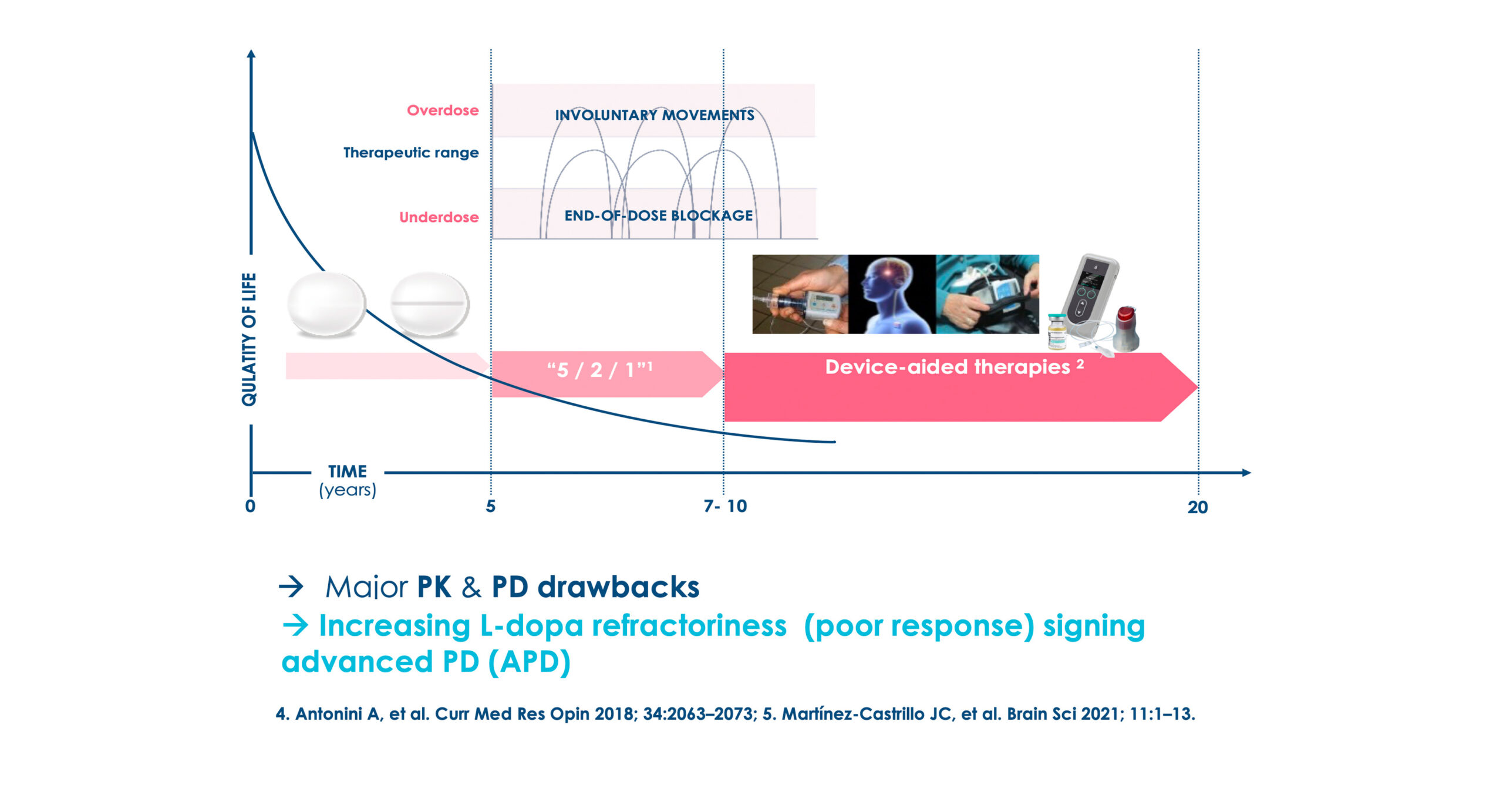
L-dopa: Backbone PD therapy
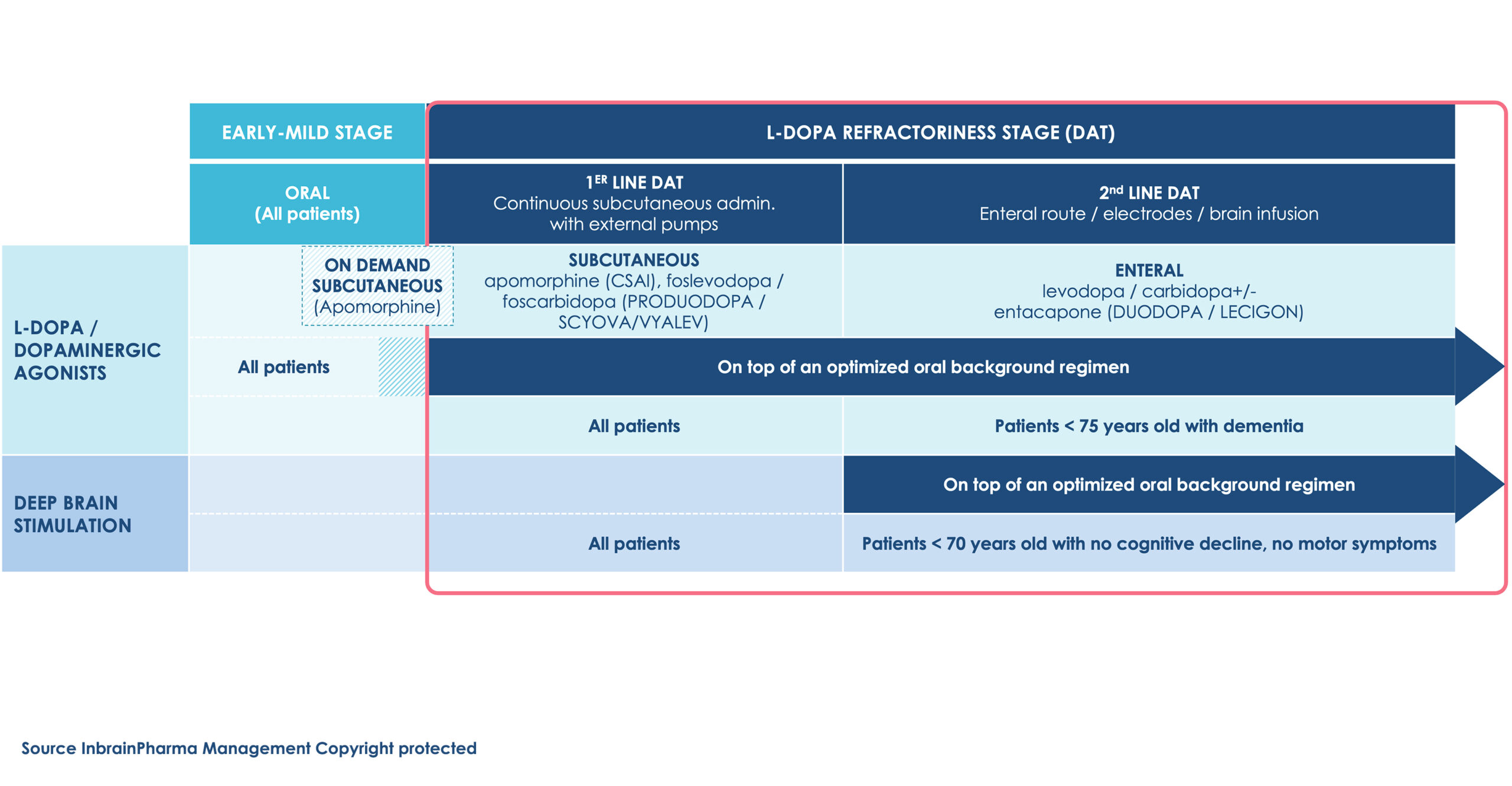
L-dopa based Device-Aided Therapies (DAT)
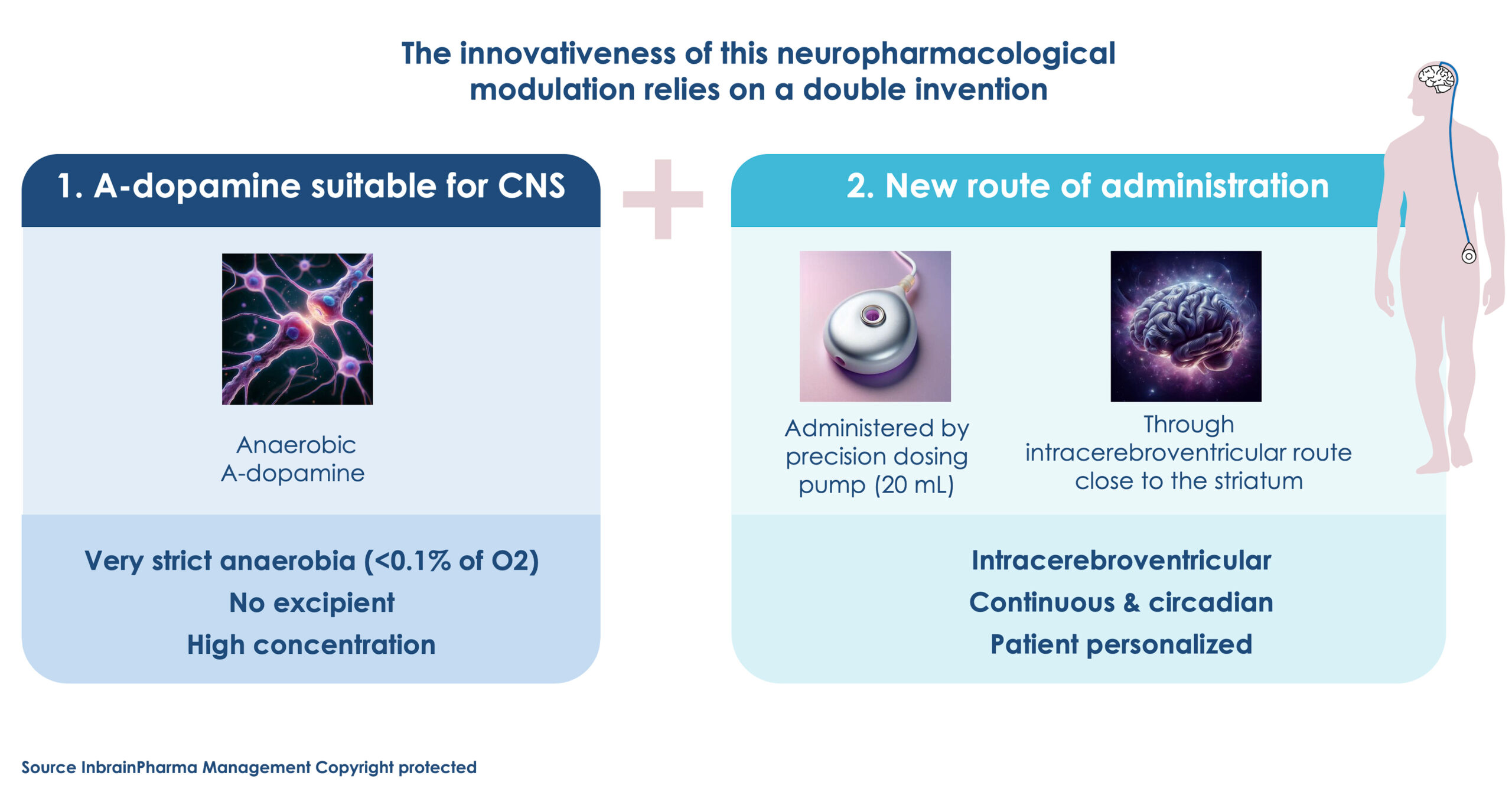
A-Dopamine
An innovative therapy for pd patients refractory to L-dopa treatments
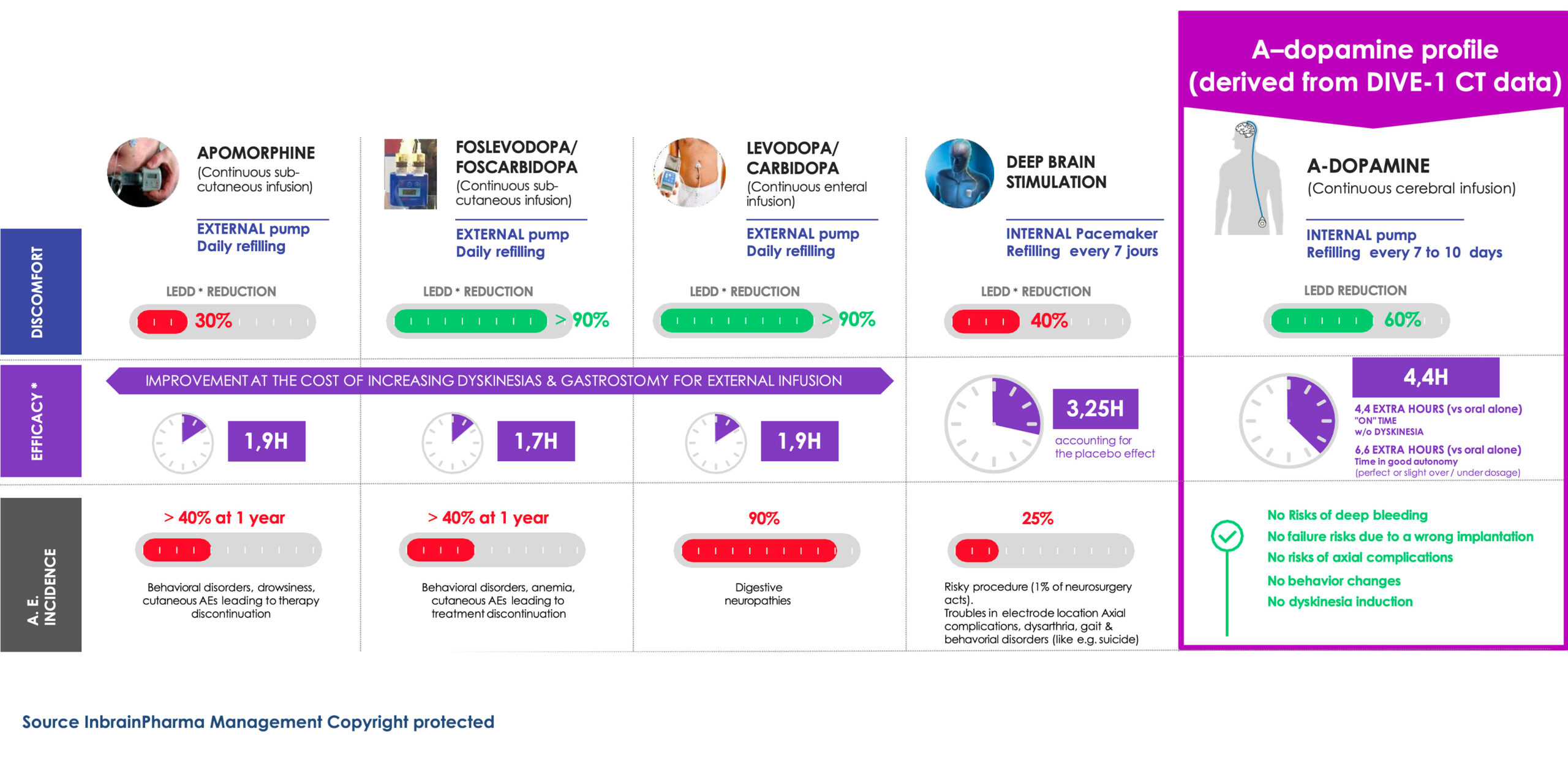
Overview of the current DAT market
Indication & positioning (EMA endorsed)
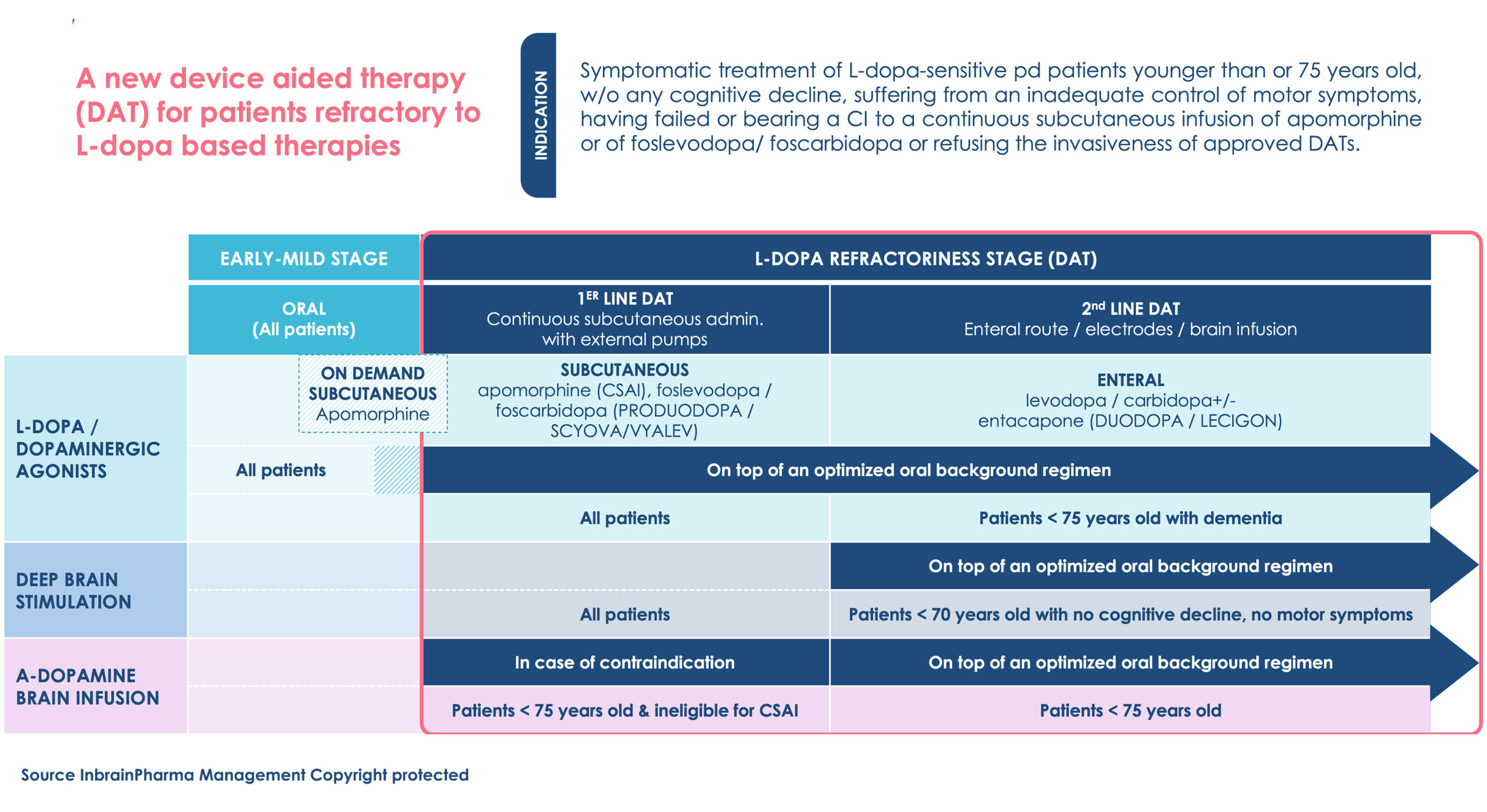
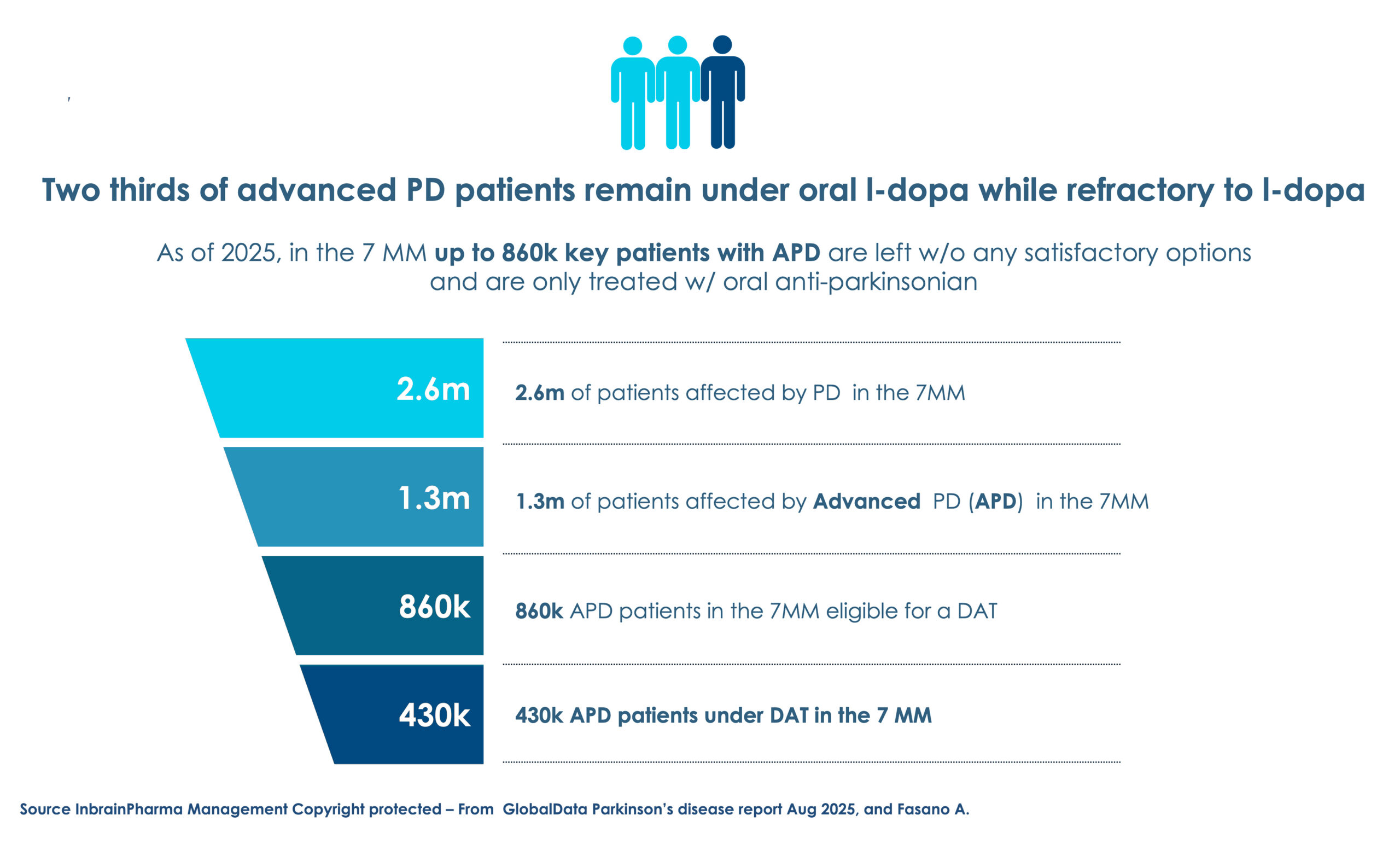
A solution addressing a high unmet need
Pipeline
Investors

InBrainPharma is currently open to collaboration with industrial and/or financial partners on the next phases of its development, please contact us.
News & events
Press Releases
Careers
There are currently no open positions in the InBrainPharma team.






















